Researchers found an ultrafast alternative to the slow oxide ion transfers of conventional fuel cells, increasing efficiency and performance by using hydrocarbon fuel directly. The commercial applications are promising.
Like batteries, fuel cells produce energy through an electrochemical process. Unlike batteries, they don’t run down or require recharging. However, the potential advantages of fuel cells are offset by challenges that include cost, performance and durability.
Michigan Technological University researcher Yun Hang Hu and two graduate students, Hanrui Su and Wei Zhang, took on those challenges, changing the conventional path of a fuel cell by creating an interface between the electrolyte and melted carbonate as an ultrafast channel for oxygen ion transfer.
“This allowed us to invent an entirely new type of fuel cell, a carbonate-superstructured solid fuel cell (CSSFC),” said Hu, who holds the Charles and Carroll McArthur Endowed Chair Professorship of Materials Science and Engineering in the Department of Materials Science and Engineering at Michigan Tech.
Like other fuel cells, CSSFCs have a wide array of potential uses, from providing energy to operate fuel cell vehicles and home power generation to entire power stations. Because CSSFCs are fuel flexible, they offer higher durability and energy conversion efficiency at lower operating temperatures than other types of fuel cells.
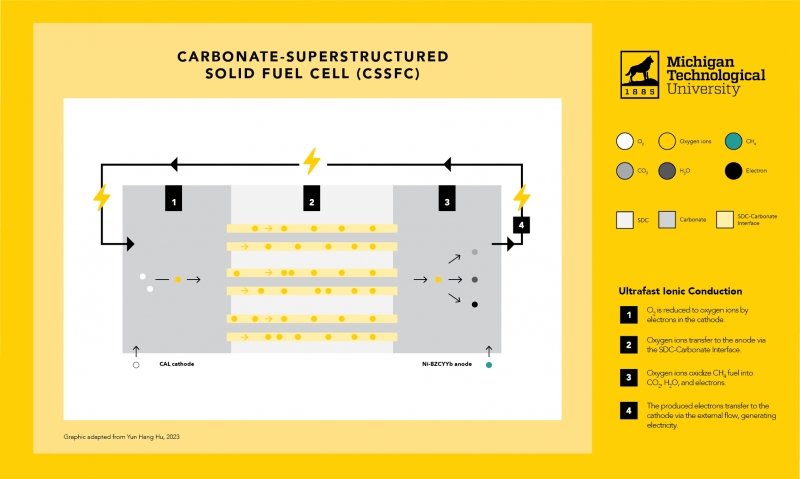
Most fuel cells are powered by hydrogen — typically produced from hydrogen-containing compounds, most often methane — via an expensive process called reforming. But the CSSFC developed in Hu’s lab can directly use methane or other hydrocarbon fuels.
Hu said fuel flexibility is of particular interest for commercial applications. And, the new fuel cell’s electrochemical performance at lower operating temperatures offers several other advantages. “The operating temperature of a conventional solid oxide fuel cell is usually 800 degrees Celsius or higher, because ion transfer in a solid electrolyte is very slow at a lower temperature,” Hu said. “In contrast, the CSSFC’s superstructured electrolyte can provide a fast ion transfer at 550 degrees Celsius or lower — even as low as 470 degrees Celsius.”
Communicating the Research
Hu, Su and Zhang had their findings, “Carbonate-superstructured solid fuel cells with hydrocarbon fuels,” published in the Proceedings of the National Academy of Sciences journal.
Most fuel cells are powered by hydrogen — typically produced from hydrogen-containing compounds, most often methane — via an expensive process called reforming. But the CSSFC developed in Hu’s lab can directly use methane or other hydrocarbon fuels.
Hu said fuel flexibility is of particular interest for commercial applications. And, the new fuel cell’s electrochemical performance at lower operating temperatures offers several other advantages. “The operating temperature of a conventional solid oxide fuel cell is usually 800 degrees Celsius or higher, because ion transfer in a solid electrolyte is very slow at a lower temperature,” Hu said. “In contrast, the CSSFC’s superstructured electrolyte can provide a fast ion transfer at 550 degrees Celsius or lower — even as low as 470 degrees Celsius.”
"The CSSFC can be directly operated with methane and various other hydrocarbon fuels — no reformation required. It’s promising for commercial applications due to this fuel flexibility."
The relatively low operating temperature offers high theoretical efficiency and lower cell fabrication costs. Hu said it is also potentially safer to operate than other solid fuel cells.
Tests on the CSSFC also showed an unprecedentedly high open circuit voltage (OCV), which indicates no current leakage loss and high energy conversion efficiency.
Hu estimates that CSSFC fuel efficiency could reach 60%. By comparison, the average fuel efficiency of a combustion engine ranges between 35% and 30%. The CSSFC’s higher fuel efficiency could lead to lower carbon dioxide emissions in vehicles.
Hu and his team began looking at methods to improve solid oxide fuel cells (SOFCs) four years ago. In addition to addressing issues associated with SOFC operating temperatures — an industrywide goal — they examined the unique properties of superstructured materials. Created with specific forms to serve specific functions, SOFCs have wide applications in science and engineering.
Lower operating temperatures with hydrocarbon fuels are problematic because they result
in slow fuel oxidation and cause coking — that’s when accumulated carbon deposits
gunk up fuel cells, degrading efficiency and performance. “Hydrocarbon oxidation kinetics
are extremely sluggish at lower temperatures due to the strong carbon-hydrogen bonds,”
Hu said. “Carbon deposition also deactivates the electrodes by covering their catalytic
sites.”
To test their hypothesis, researchers fabricated a device in the lab. "In our experiments,
the CSSFC exhibited ultrahigh oxygen ionic conductivity at 550 degrees Celsius, achieving
rapid oxidation of hydrocarbon fuel. This led to an unprecedented high open-circuit
voltage of 1.041 volts and a very high peak power density of 215 milliwatts per square
centimeter, along with excellent coking resistance using dry methane fuel," said Hu.
He and his team will continue to look to new frontiers, including creating superstructured
materials as a new platform for energy devices.
"Finally, our observations stimulated a hypothesis: A continuous interface between molten carbonate and a solid ionic conductor could constitute an ultrafast transfer channel for oxygen ions — namely, an oxygen ionic superconductor."
Michigan Technological University is an R1 public research university founded in 1885 in Houghton, and is home to nearly 7,500 students from more than 60 countries around the world. Consistently ranked among the best universities in the country for return on investment, Michigan's flagship technological university offers more than 185 undergraduate and graduate degree programs in science and technology, engineering, computing, forestry, business, health professions, humanities, mathematics, social sciences, and the arts. The rural campus is situated just miles from Lake Superior in Michigan's Upper Peninsula, offering year-round opportunities for outdoor adventure.





Comments